The role a catalyst plays in increasing the reaction rate.
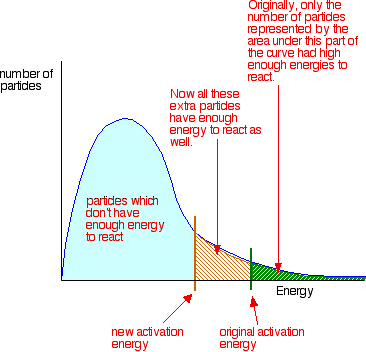
The presence of a catalyst increases the reaction rate (in both the forward and reverse reactions) by providing an alternative pathway with a lower activation energy but is chemically unchanged at the end of the reaction. When the reaction has finished, you would have exactly the same mass of catalyst as you had at the beginning.
Role of activation energy in a chemical reaction.
Activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius, that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction. The activation energy of a reaction is usually denoted by Ea, and given in units of kilojoules per mole.
Activation energy can be thought of as the height of the potential barrier (sometimes called the energy barrier) separating two minima of potential energy (of the reactants and products of a reaction). For a chemical reaction to proceed at a reasonable rate, there should exist an appreciable number of molecules with energy equal to or greater than the activation energy.

 The sparks generated by striking steel against a flint provide the activation energy to initiate combustion in this Bunsen burner. The blue flame will sustain itself after the sparks are extinguished because the continued combustion of the flame is now energetically favorable.
The sparks generated by striking steel against a flint provide the activation energy to initiate combustion in this Bunsen burner. The blue flame will sustain itself after the sparks are extinguished because the continued combustion of the flame is now energetically favorable.


No comments:
Post a Comment